Chemical patterns
The evolution of the atom
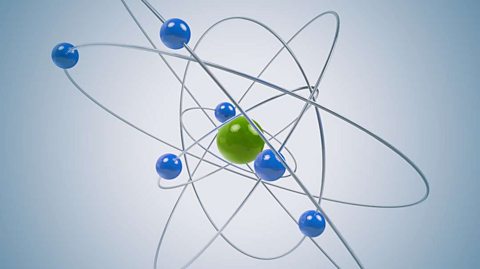
The atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated from its atomic number and mass number.
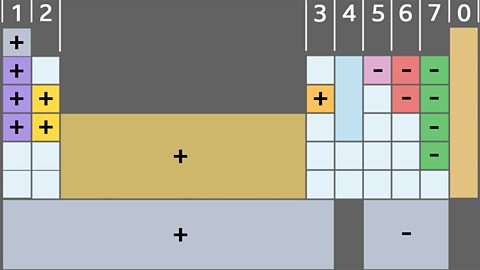
Periodic table of elements
Mendeleev made an early periodic table. In the modern periodic table, elements are arranged in order of atomic number in periods and groups. Electronic arrangements model how electrons are arranged.

Metals, non-metals and compounds
In this OCR GCSE Chemistry study guide, we'll go through the group 0 elements, the noble gases, are all unreactive non-metal gases. We'll explain how they show trends in their physical properties and how their uses depend on their inertness, low density and non-flammability.

Using equations to represent chemical reactions
All substances are described by their formulae, which are used to write balanced chemical equations. Writing the formula for an ionic compound requires knowledge of the charges on its ions.
Properties of transition metals
Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what purpose.

Sample exam questions - chemical patterns
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link