The nature of substances and chemical reactions
Elements, compounds and formulae
An overview of the definitions of different types of chemicals, how chemicals can be represented in chemical equations, how chemicals can be separated, and a number of important calculations related to chemical formulae.

Separating mixtures
Different experimental techniques are used to separate a variety of mixtures into individual substances. There are also a number of ways to distinguish physical and chemical changes.

Chemical equations and calculations
Reaction information is shown using word and symbol equations. Mass is conserved in chemical reactions. We can predict the masses of products and reactants involved in chemical reactions as no atoms are made or destroyed in chemical reactions. Relative atomic masses can be used to find the relative formula mass of a compound.
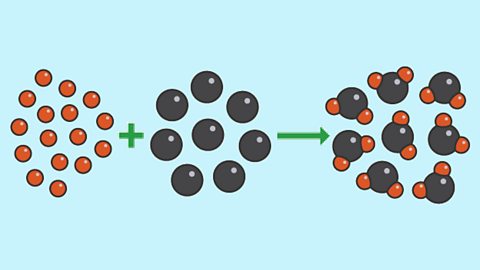
Further calculations [Higher tier only]
Calculation techniques for the higher tier to determine the empirical and molecular formulae of a substance, masses of a required substance in a reaction, and calculations involving moles.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link