Chemical changes and structures
Rates of reaction
The speed of a chemical reaction is affected by temperature, concentration, particle size and the presence of a catalyst. It can be calculated by measuring changes in reactants/products.

Atomic structure
Atoms are made from protons, neutrons and electrons. In this study guide, you can revise how the periodic table arranges elements according to their atomic size and other properties, atomic theory and atomic numbers, you'll also learn about isotopes and the properties of the main groups of elements.
Bonding and properties of materials
Only the noble gases exist as individual atoms not bonded to other atoms. In all other substances atoms are held together by chemical bonds, either sharing or gaining/losing electrons.

Chemical formulae
The chemical formula for a substance shows how many atoms of each element are present in a molecule, or the proportion of atoms of each element. The formula can be worked out using the valency.

Balanced equations
Chemical equations must be balanced so that the quantities of reactants and products match. For an equation to be balanced there must be an equal numbers of atoms on each side.

The mole and concentration of solutions
The gram formula mass of a substance is known as the mass of one mole. The mass, number of moles, concentration or volume of a substance can be calculated easily if you learn two formula triangles.

Acids and bases
The pH scale measures the acidity or alkalinity of a solution. A pH less than 7 is acidic. Alkalis dissolve in water to give a pH greater than 7. A pH equal to 7 indicates a neutral solution.

Video playlist
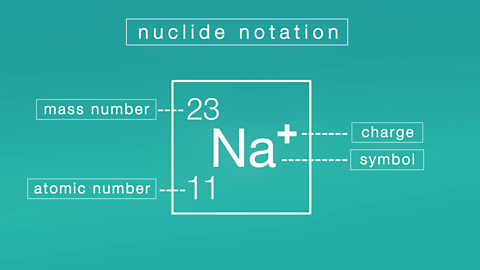
Structure of an ion. Video
Learn about the basic structure of an ion, related to atomic number and mass.
Titration. Video
Calculate the concentration of an acid or alkali using the process of titration

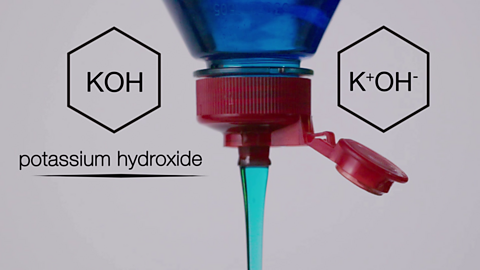
Ionic compounds. Video
Ionic compounds, structures, bonding and properties.

Ionisation. Video
Ions are electrically charged particles that form when atoms lose or gain electrons.

Atoms and isotopes. Video
An introduction to how protons, neutrons and electrons make up atoms.

Atomic structure. Video
Learn about the basic structure of atom, atomic numbers and mass.

Acids, alkalis and bases. Video
The PH scale measures the level of a solution's acidity or alkalinity.
Creating a balanced equation. Video
Learn how to write balanced equations.

Rates of reaction. Video
Learn how to measure the rate of a reaction and about the factors that affect it.

Calculations from balanced equations. Video
Calculate the mass of reactants and products from a thermite reaction.

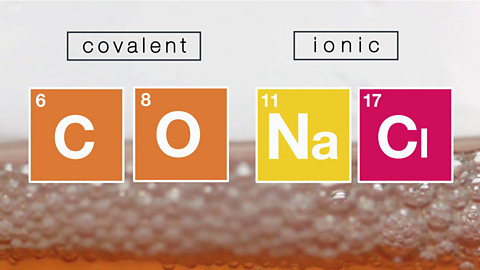
Covalent and ionic compounds. Video
Learn how to work out if a bond is covalent and ionic.
The mole. Video
Chemists predict the weight of chemical substances using a unit called 'the mole'
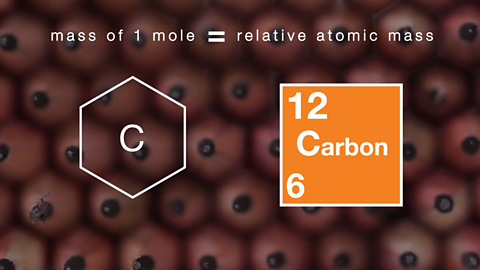
How mass and atomic numbers explain atomic structure. Video
Atomic and mass numbers show how many protons, neutrons and electrons there are in atoms.

Links
- External linkExternal link
- External linkExternal link
- External linkExternal link
- External linkExternal link