Elements, compounds and mixtures
Separating mixtures - OCR Gateway
There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. The method chosen depends upon the type of mixture.

Periodic table - OCR Gateway
Mendeleev made an early periodic table. In the modern table, elements are put in order of atomic number into periods and groups. Electron arrangements model how electrons are arranged in atoms.

Chemical bonding - OCR Gateway
Ionic bonds, covalent bonds and metallic bonds are examples of chemical bonds. The structure and bonding in a substance are modelled in different ways, including dot and cross diagrams.
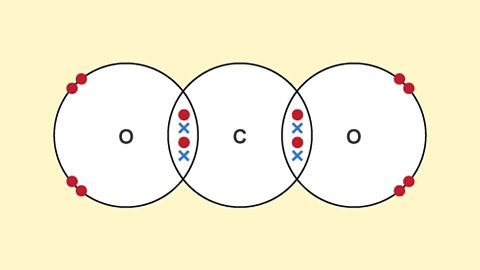
Properties of materials - OCR Gateway
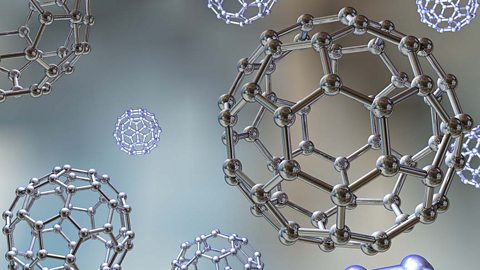
Carbon atoms can form four covalent bonds. This is why they can form many different organic substances, such as diamond, graphite and fullerenes. Different substances have different bulk properties.
Nanoparticles - OCR Gateway
Nanoparticles are 1 nm to 100 nm in size. They have very large surface area to volume ratios. The properties of nanoparticulate substances are different from those of the same substance in bulk.
Sample exam questions - OCR Gateway
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link