Contraceptive pills recalled in South Africa after mix-up
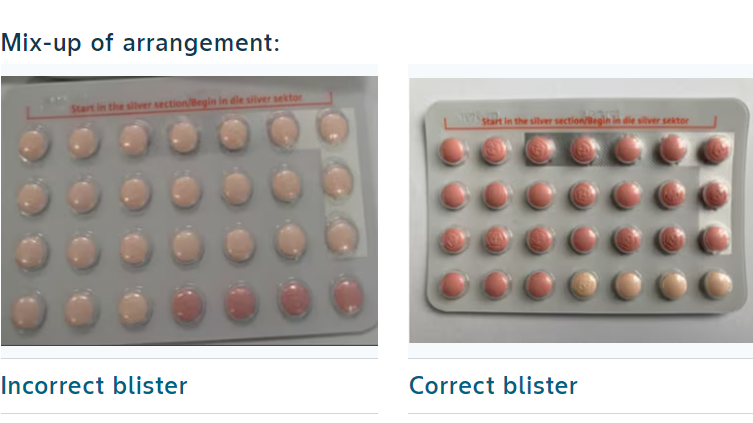
The pills were organised incorrectly and contained the wrong number of active pills
- Published
Regulators in South Africa have recalled a batch of the popular Yaz Plus contraceptive pill, after a packaging mix-up which means the contraception could be potentially ineffective.
Manufacturer Bayer Ltd said women using pills from the affected batch should stop immediately and seek medical advice.
A packaging mix-up led to a number of blister packs carrying 24 inactive pills, instead of 24 hormone-containing active pills.
The issue affected only a limited number of packets in a specific batch labelled WEW96J, expiring in March 2026
The erroneous batch has been recalled by Bayer, , with the company stressing the "root cause" of the mix-up had been identified and dealt with.
A regular pack of Yaz Plus contraceptives contains 24 active pills containing hormones, which are pink in colour, followed by four hormone-free, inactive pills, which are light orange in colour.
In the recalled batch, a number of packs instead carried 24 hormone-free inactive pills and only four active hormone pills.
The concern is that a woman could be at risk of becoming pregnant having taken inactive pills believing she was taking effective hormonal contraception.
says: "While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your healthcare practitioner, as they may potentially not provide the contraceptive protection you expect."
Anyone who has acquired a packet of pills belonging to the stipulated batch is advised to return the tablets to pharmacies for a replacement or refund.
Healthcare professionals, hospitals, pharmacies, doctors, nurses and wholesalers who have packets of the affected batch should also return them.
In a statement, Bayer Ltd said "the root cause for the mix-up of tablets in the packaging has been identified and corrective measures have been implemented".
The incident is limited to only one batch and no other batches are affected, the company said.
The company has set up a .