Atoms
Everything is made from atoms, including you. Atoms are tiny particles that are far too small to see, even with a microscope.To make diagrams simpler we often draw atoms as circle
Elements
There are lots of different substances but only just over a hundred elements.
An element is a substance that is made up of only one kind of atom.
The atoms in a particular element are the same as each other, and they are different from the atoms of all other elements. For example, copper and gold are elements. A piece of gold contains only gold atoms. A piece of copper contains only copper atoms.

There are 118 known elements. 94 occur naturally on Earth, the rest can be made by nuclear reactions.
The atoms of some elements, like copper, do not join together and instead exist on their own as individual atoms and are not molecules.
The atoms of other elements, however, like hydrogen, join with another hydrogen atom as a pair, making a molecule.
Chemical symbols
Each element is given the chemical symbol of its atom. H is the symbol of the hydrogen atom O for oxygen. Chemical symbols are usually one or two letters long.Every chemical symbol starts with a capital letter, with the second letter written in lower case.For example, Mg is the correct symbol for magnesium, but mg, mG and MG are wrong. Take care to write chemical symbols correctly.
Symbols and names
Sometimes it is easy to tell which element a symbol stands for. For example, C stands for carbon and Li stands for lithium.
But sometimes the symbol comes from a name for the element that is not an English word. For example, Lead is a chemical element with the symbol Pb (from the Latin for lead, plumbum).W stands for tungsten (from the word wolfram) and Na stands for sodium (from the word natrium).
The same chemical symbols are used all over the world, no matter which language is spoken, which makes them very useful.
Some common elements
| Element | Symbol | Appearance | Use |
|---|---|---|---|
| aluminium | Al | Light, non-corroding, shiny, silver-grey metal solid | In cans, aeroplane parts, pots and pans |
| carbon | C | A black or dark brown, soot-like powder or a black, shiny solid which comes off when moved over paper | Graphite is used for pencils and electrodes. Diamonds in jewellery. Coal, oil, gas and feedstock |
| chlorine | Cl | A green, poisonous gas | As an antiseptic in swimming pools. Production of paper |
| copper | Cu | Bronze coloured soft metal | Electric wires, motors and generators. Radiators, and heating systems |
| gold | Au | Dense, non-tarnishing, coloured metal solid | Used in jewellery and coins, gears for watches, artificial limb joints |
| hydrogen | H | An explosive gas which has no colour or smell | Liquid fuel ÔÇô the main fuel for the Space Shuttle |
| iron | Fe | Dull grey, solid, medium-hard metal. Magnetic | To make steel. Electromagnets in scrap yards and doorbells |
| mercury | Hg | Silver coloured liquid metal, toxic | Used in thermometers, barometers and electrical switches. Streetlights and fluorescent lamps |
| nitrogen | N | A colourless gas which has no smell | Found in air ÔÇô 78 % of Earth's atmosphere is made up of nitrogen. Fertilizers, ammonia and nylon |
| oxygen | O | A colourless gas which has no smell | Is necessary for respiration. Aids burning. Found in air. Used to treat breathing problems |
| sulfur | S | A bright yellow, brittle solid | In match heads, gunpowder and fireworks, rubber manufacture |
Compounds
A compound is a substance that contains atoms of two or more different elements, chemically joined together.
For example, water is a compound of hydrogen and oxygen, chemically joined together. Each of its molecules contains two hydrogen atoms and one oxygen atom. There are very many different compounds and all compounds are molecules.
Carbon dioxide is a compound of carbon and oxygen, chemically joined together. Each of its molecules contains two oxygen atoms and one carbon atom
What is a compound?
Summary
- There are many substances but only 118 known elements
The atoms of some elements, like copper, do not join together and instead exist on their own as individual atoms and are not molecules.
The atoms of other elements, however, like hydrogen, join with another hydrogen atom as a pair, making a molecule.
So, all compounds are molecules, and some elements are too.
The periodic table
All the different elements are arranged in a chart called the periodic table. A Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables in the 1869. At that time, he had only 50 elements to arrange.
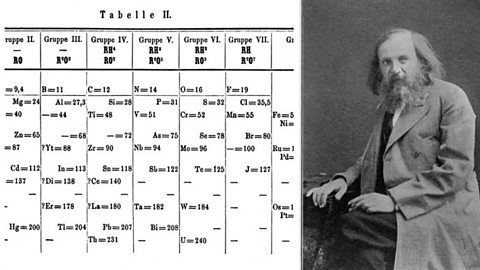 Image source, SSPL/PRINT COLLECTOR
Image source, SSPL/PRINT COLLECTORThe modern periodic table is based closely on the ideas he used:
- the elements are shown by the symbol for their atom
- the elements are arranged in mass order
- the horizontal rows are called periods
- the vertical columns are called groups
- elements in the same group (same vertical column) have similar chemical properties
Some things to look for in the periodic table
The main groups are numbered from 1 to 7 going from left to right, and the last group on the right is group 0.
The zig-zag line in this diagram separates the metals, on the left, from non-metals, on the right. Hydrogen is a non-metal, but it is often put in the middle.
Notice that most elements are metals, rather than non-metals.
The only liquid elements at room temperature are bromine (Br) and mercury (Hg)
Eleven non-metals are gases at room temperature, including oxygen and chlorine (see more below in non-metals)The section in the middle of the table is called the Transition Metals. Many common metals are found here such as gold, Au, silver Ag, copper, Cu and iron, Fe.
Transition metals are found between groups 2 and 3.
Remember that you will only find elements in the periodic table and never compounds. So, you wonÔÇÖt find substances like water or copper sulfate in the periodic table.
Making predictions using the periodic table
If you know what one of the elements in a group is like, you can make predictions about the other elements in a group. For example, all the elements in group 1 are reactive metals, and all the elements in group 0 are unreactive non-metals.
There are usually trends in properties that allow us to make further predictions.For example, in group 1:
| Melting point | Density | Reactivity | |
|---|---|---|---|
| Lithium | Decreases down the group | Increases down the group | Increases down the group |
| Sodium | Decreases down the group | Increases down the group | Increases down the group |
| Potassium | Decreases down the group | Increases down the group | Increases down the group |
| Rubidium | Decreases down the group | Increases down the group | Increases down the group |
Caesium is the next element in group 1, and it can be found below rubidium. You can accurately predict that it will have the lowest melting point, the highest density and the highest reactivity of all the elements in group 1.
Metals
Iron, magnesium and gold are examples of metal elements. Metals have properties in common.
They are:
- shiny and lustre, especially when they are freshly cut
- good conductors of heat and electricity
- malleable which means they can be bent and shaped without breaking
Most metals also have other properties in common. They are:
- solid at room temperature, except mercury
- hard and strong
- they have a high density
- they are sonorous, which means they make a ringing sound when hit ÔÇô thatÔÇÖs why bells are made from metal

Three metals (iron, cobalt and nickel) are magnetic. Steel is a mixture of elements but it is mostly iron, and it is also magnetic. The other metal elements are not magnetic.
Reactions of metals
Metals react with oxygen to produce compounds called metal oxides. For example, magnesium reacts with oxygen to produce magnesium oxide. The reaction can be represented by this word equation:
magnesium + oxygen  magnesium oxide
Metal oxides are bases. They react with acids and neutralise them. Some metal oxides dissolve in water and, when they do, they produce alkaline solutions.
Non-metals
Oxygen, carbon, sulfur and chlorine are examples of non-metal elements. Non-metals have properties in common. They are:
- dull (not shiny)
- poor conductors of heat and electricity (they are insulators)
- weak and brittle (they easily break or shatter when solid)
Most non-metals also have these properties:
- they have a low density (they feel light for their size)
- they are NOT sonorous - they do not make a ringing sound when hit
Eleven of the non-metal elements are gases at room temperature. Hydrogen, H, nitrogen, N, oxygen, O, fluorine, F, chlorine, Cl, and all the noble gases of Group 0, helium, He, neon, Ne, argon, Ar, krypton, Kr, xenon, Xe, and radon, Rn, are all gases at room temperature.
One non-metal, bromine Br, is a liquid at room temperature.The other non-metals are solids at room temperature, including carbon and sulfur.
Reactions of non-metals
Non-metals react with oxygen to produce non-metal oxides. For example, sulfur reacts with oxygen to produce sulfur dioxide. The reaction can be represented by this word equation:
sulfur + oxygen  sulfur dioxide
Non-metal oxides react with bases and neutralise them. Some non-metal oxides dissolve in water and, when they do, they produce acidic solutions.
Diamond and graphite
Carbon is a solid non-metal element. Pure carbon can exist in very different forms. The most common two are diamond and graphite. The table shows some differences between them.
| Diamond | Graphite |
|---|---|
| Transparent and colourless | Opaque and black |
| Hard | Soft |

Diamond is the hardest natural substance on Earth, but it is also very brittle and will shatter if hit with a hammer.
Graphite is unusual because it is a non-metal that conducts electricity.
Metals v non-metals
About three quarters of elements are metals, rather than non-metals. The table summarises some differences in their properties.
Properties
| Metals | Non-metals | |
|---|---|---|
| Appearance | Shiny | Dull |
| State at room temperature | Solid (except mercury, which is a liquid) | About half are solids, about half are gases, and one (bromine) is a liquid |
| Density | High (they feel heavy for their size) | Low (they feel light for their size) |
| Strength | Strong | Weak |
| Malleable or brittle | Malleable (they bend without breaking) | Brittle (they break or shatter when hammered) |
| Conduction of heat | Good | Poor (they are insulators) |
| Conduction of electricity | Good | Poor (they are insulators, apart from graphite) |
| Magnetic material | Only iron, cobalt and nickel | None |
| Melting point | Usually high | Usually low |
| Boiling point | Usually high | Usually low |
| Sound when hit | They make a ringing sound - they are sonorous | They make a dull sound |
| Type of oxide | Basic or alkaline | Acidic |
Telling them apart
Notice that metals and non-metals have opposite properties to each other. It is usually easy to tell metals and non-metals apart, but some tests are more reliable than others.
For example, using a magnet is not a good test to see if an element is a metal. This is because only three metals are magnetic.
Pure chemical substances

Food and drink may be advertised as ÔÇÿpureÔÇÖ. For example, you may see cartons of ÔÇÿpure orange juiceÔÇÖ or ÔÇÿpure mineral waterÔÇÖ. This means that nothing else was added to the orange juice or mineral water during manufacture. However, these substances are not pure to a scientist.

In science:
- a pure substance contains only one element or compound.
Mineral water is mostly water, but there are other substances mixed with it. These are the ingredients that you see listed on the bottleÔÇÖs label

It is difficult to get completely pure substances ÔÇô there will almost always be other substances mixed in. Even the purest water will contain dissolved gases from the air. Impurities in a substance will affect its properties. For example, they may change its boiling point.
Mixtures
A mixture contains different substances that are not chemically joined to each other. For example, a packet of sweets may contain a mixture of different coloured sweets. The sweets are not joined to each other, so they can be easily picked out and separated into piles.
Air is a mixture of gases. The amount of water vapour in the air varies from place to place, and day to day. For this reason, the proportions of the gases in the air are usually given for dry air.
Some of the gases in the air are elements:
- nitrogen, \(N_2\)
- oxygen, \(O_2\)
- argon, Ar
Some are compounds (see below), including:
- carbon dioxide, \(CO_2\)
- water vapour, \(H_2\)O
| Gas | Symbol | Element or compound | % of gas in dry air |
|---|---|---|---|
| Nitrogen | \(N_2\) | Element | 78 |
| Oxygen | \(O_2\) | Element | 21 |
| Argon | Ar | Element | 0.9 |
| Carbon dioxide | \(CO_2\) | Compound | 0.04 |
| Other gases | Element and Compound | 0.06 |
This information can be represented in a pie chart:
Dissolving
Dissolving is one way to make a mixture. For example, when salt is stirred into water, the salt dissolves in the water to make salt solution. In a solution:
- the substance that dissolves is called the solute
- the liquid that the solute dissolves in is called the solvent
- the liquid mixture of solute and solvent is called the solution
In salt solution, salt is the solute and water is the solvent. The particles of solute and solvent are completely and evenly mixed. Solutions are clear ÔÇô you can see through them.
Common solvents include water, ethanol and white spirit.
Science presenter Jon Chase defines the terms solute, solvent and solution
Diffusion is another way to make a mixture. Particles of different substances mix together during diffusion. This happens:
- quickly in gases, because their particles are able to move quickly in all directions
- slowly in liquids, because their particles can only move around each other, and they can push other particles around
- not at all in solids because their particles can only vibrate on the spot
Compounds
A compound contains atoms of different elements, chemically joined together.
Compounds form in chemical reactions, and you need other chemical reactions to separate a compound into its elements. The diagrams show what happens when iron filings and sulfur powder react together in a chemical reaction, rather than just mix together.
Properties of mixtures and compounds
Mixtures have different properties from compounds. The table summarises these differences:
| Mixture | Compound | |
|---|---|---|
| Composition | Variable composition ÔÇô you can vary the amount of each substance in a mixture | Definite composition ÔÇô there is a fixed amount of each element in a compound |
| Joined or not | The different substances are not chemically joined together | The different elements are chemically joined together. Often, heat is given out or taken in when a compound is formed |
| Properties | Each substance in the mixture keeps its own properties | The compound has properties different from the elements it contains |
| Separation | Each substance is easily separated from the mixture | Difficult to separate into its elements ÔÇô can only be done using chemical reactions |
| Examples | Air, sea water, most rocks | Water, carbon dioxide, magnesium oxide, sodium chloride |
Summary: chemical substance
Notice that the different substances in a mixture can be single atoms, molecules of elements or molecules of compounds.
Question
Which of the following is a compound?
- Air
- Water
- Oxygen
Water is a compound because it is made up of two or more different elements, chemically joined together.
Question
Which one of the following is a mixture?
- carbon dioxide
- air
- hydrogen
Air is a mixture as it contains two or more substances that are not joined together and are easily separated.
Question
Which one of the following is an element?
- carbon dioxide
- water
- helium
Helium is an element as it is a substance that is made up of only one kind of atom and cannot be broken down into any other, simpler substance
More on Chemistry
Find out more by working through a topic
- count5 of 8
- count6 of 8

- count7 of 8

- count8 of 8
