The periodic table
Copper has been used by humans for as much as 7000 years and elements such as gold, silver, tin, lead and mercury have been known for many thousands of years. However, the first scientific discovery of an element occurred in 1649 when Hennig Brand discovered phosphorous. The discovery of other elements followed regularly and soon it became necessary to arrange them in some sort of order.
The earliest attempt to classify the elements was in 1789, when Antoine Lavoisier grouped the elements based on their properties into gases, non-metals, metals and earths. Several other attempts were made to group elements together over the coming decades.
Then in 1869, a Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables. He wrote the properties of the elements on pieces of card and rearranged them until he realised that, by putting them in order of increasing atomic weight, certain properties of elements regularly occurred. At that time, he had only 50 elements to arrange.
Not only did Mendeleev arrange the elements in the correct way, but he also had the foresight to leave gaps for undiscovered elements. Over time these gaps have gradually been filled in as scientists unearthed new elements. For instance they discovered phosphorus when they isolated it from urine.
Mendeleev never received a Nobel Prize for his work, but element 101 was named Mendelevium, Md, after him. Not to miss out, element 102, Nobelium, No, is named in honor of Alfred Nobel, who set aside his vast fortune to establish Nobel Prizes.
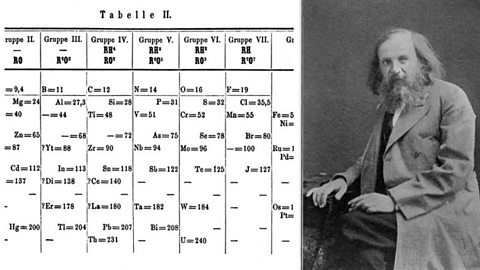 Image source, SSPL/Print collector
Image source, SSPL/Print collectorThe modern periodic table is based closely on the ideas he used:
- the elements are shown by the symbol for their atom
- the elements are arranged in mass order (order of increasing atomic number)
- the horizontal rows are called periods
- the vertical columns are called groups
- elements in the same group (same vertical column) have similar chemical properties
- there are 118 known elements
The main groups are numbered from 1 to 7 going from left to right, and the last group on the right is group 0. The section in the middle of the table is called the Transition Metals.
If you know what one of the elements in a group is like, you can make predictions about the other elements in a group. For example, all the elements in group 1 are reactive metals, and all the elements in group 0 are unreactive non-metals.
The zigzag, staircase, line in the diagram of the periodic table above separates the metals, on the left, from non-metals, on the right. Hydrogen is a non-metal, but it is often put in the middle.
Notice that most elements are metals, rather than non-metals. In fact, nearly 75% of elements are metals.
Elements and symbols
You will remember from the Chemical study guide that:
An element is a substance that is made up of only one kind of atom and cannot be broken down into any other, simpler substance
A simple shorthand system is used for representing elements. The symbol for the element is the first letter of its English or Latin name, written as a capital letter. If several elements have the same first letter, a second, lower case letter is added.
A list of some common elements and their symbols is shown below
| Element | Symbol |
|---|---|
| Aluminium | Al |
| Argon | Ar |
| Bromine | Br |
| Calcium | Ca |
| Carbon | C |
| Chlorine | Cl |
| Cobalt | Co |
| Copper | Cu |
| Fluorine | F |
| Gold | Au |
| Helium | He |
| Hydrogen | H |
| Iodine | I |
| Iron | Fe |
| Lead | Pb |
| Magnesium | Mg |
| Mercury | Hg |
| Neon | Ne |
| Nitrogen | N |
| Oxygen | O |
| Phosphorus | P |
| Potassium | K |
| Silicon | Si |
| Silver | Ag |
| Sodium | Na |
| Sulfur | S |
| Tin | Sn |
| Uranium | U |
| Zinc | Zn |
Groups
Elements in the same group have similar chemical properties
| Group | Name | Metal / Non-metal | Reactive / Non-reactive |
|---|---|---|---|
| 1 | alkali metals | metal | reactive |
| 2 | alkaline earth metals | metal | reactive |
| 7 | halogens | non-metal | reactive |
| 0 | noble gases | non-metal | non-reactive |
A closer look at some of the groups
Group 1: The alkali metals
Group 1
Group 1 elements have similar physical and chemical properties:
- they are very reactive
- low density - the first three ÔÇô lithium, sodium and potassium ÔÇô are less dense than water and so float on water
- very soft - they are easily cut with a knife
- shiny when first cut, but tarnish rapidly in air
- low melting points - the melting points decrease as the atoms get bigger going down the group
The alkali metals are very reactive:
- the reactivity increases as you go down the group because the atoms become larger
- lithium, at the top of the group, fizzes with water; francium, at the bottom of the group, reacts explosively with water
- use tweezers when lifting alkali metals
- use a safety screen
- wear safety glasses and gloves
- use small pieces of metal
- they must be stored under oil to prevent them reacting with air or water vapour

Reaction with water
All Group 1 elements react with water to produce a metal hydroxide and hydrogen. The metal hydroxide produced dissolves in water to form an alkaline solution. The solutions turn universal indicator purple, showing they are strongly alkaline. This is why the group 1 elements are called alkali metals.
Remember: strong alkalis are corrosive - care must be taken when they are used, goggles and gloves should be worn.
The word equation for the reaction between sodium and water is:
sodium + water  sodium hydroxide + hydrogen
The physical properties of the group 1 alkali metals:

| Melting point | Boiling point | Density | Reactivity | |
|---|---|---|---|---|
| Lithium | Decreases down the group | Decreases down the group | Increases down the group | Increases down the group |
| Sodium | Decreases down the group | Decreases down the group | Increases down the group | Increases down the group |
| Potassium | Decreases down the group | Decreases down the group | Increases down the group | |
| Rubidium | Decreases down the group | Decreases down the group | Increases down the group | Increases down the group |
| Caesium | Decreases down the group | Increases down the group | Increases down the group |
Group 7: The halogens
All the elements in group 7:
- are non-metals
- have a distinctive or unpleasant smell
- are poisonous (toxic) - a fume cupboard is used for experiments
- are coloured
- have low melting points
- made up of molecules of two atoms e.g. bromine molecule \(Br_2\), chlorine molecule, \(Cl_2\)
- less reactive as you move down the group
| Halogen | Symbol | Colour | State at room temperature and pressure |
|---|---|---|---|
| fluorine | F | yellow | gas |
| chlorine | Cl | yellow-green | gas |
| bromine | Br | red-brown | liquid |
| iodine | I | grey-black | solid |
| astatine | At | black | solid |
The melting points and boiling points of the halogens increase going down group 7, and their colour gets darker.
Astatine is the rarest naturally occurring element in the Earth's crust.
Iodine sublimation
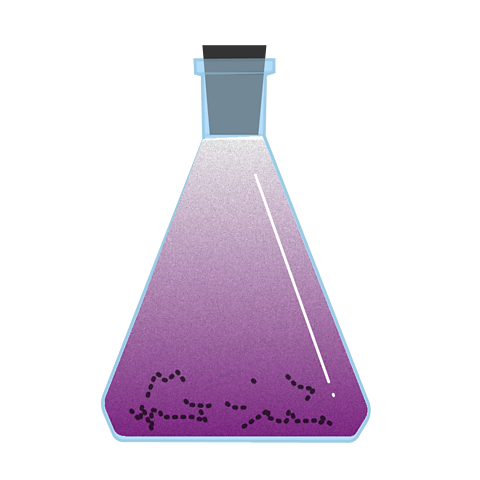
Iodine is a grey-black solid at room temperature and pressure. If heated, it sublimes to form purple iodine gas. Sublimation is the change of state from solid directly to gas on heating, without passing through the liquid phase.

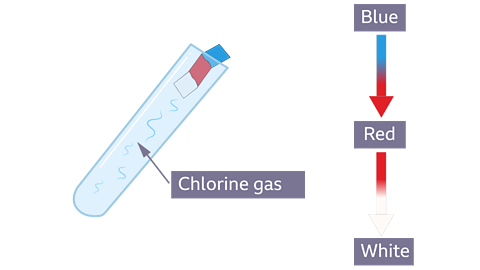
Test for chlorine gas
Chlorine has a characteristic sharp, choking smell. It also makes damp, blue universal indicator paper turn red, and then bleaches it white.
Group 0: The noble gases
The elements in group 0 are:
- very unreactive
- non-metals
- gases
- colourless
- odourless
- have low boiling points ÔÇô in fact helium, at the top of group 0, has the lowest boiling point of any element
- found in the EarthÔÇÖs atmosphere in small amounts
- used in advertising signs as they can glow with distinctive colours when a high voltage is applied across them
- formed of particles with one atom

All noble gases have low boiling points. Their boiling points increase as you move down the group. The density of the gas increases as you move down the group too.
The Group 0 elements are called noble gases because they are so ÔÇ£majesticÔÇØ that, in general, they don't react with anything. For this reason, they are also known as inert gases.
Transition metals
The transition metals are in the block in the middle of the Periodic Table, between Groups 2 and 3. They are all metals and include many common metals such as chromium (Cr), iron (Fe), nickel (Ni) and copper (Cu).
The transition metals are:
- all metals
- high density
- conductors of electricity
- conductors of heat
- have high melting point (except mercury which is liquid at room temperature)
- often form coloured compounds
The table below compares the physical properties of the transition metals and the alkali metals.
| Physical properties | Group 1 ÔÇô alkali metals | Groups 2 and 3 ÔÇô transition metals |
|---|---|---|
| Melting point | low melting point | high melting point (except mercury) |
| Density | low density ÔÇô Li, Na and K are less dense than water | high density |
| Reactivity with water | very reactive with cold water Group 1 metal + water  metal hydroxide + hydrogen | low reactivity with water some react with steam to form an oxide and hydrogen copper does not even react with steam |
| Colour of compounds | form white compounds, e.g. sodium chloride is white | form coloured compounds (e.g. copper(II) carbonate is green) |
Some of the coloured compounds:
| Transition metal compound | copper(II) oxide | copper(II) carbonate | hydrated copper(II) sulfate | any copper(II) salt in solution |
|---|---|---|---|---|
| Colour | black solid | green solid | blue crystals | blue solution |
Making predictions using the periodic table
Groups in the periodic table contain elements with similar properties. The trends in properties allow scientists to make predictions.
For example:
the group 1 alkali metals are:
| Melting point | Density | Reactivity | |
|---|---|---|---|
| Lithium | Decreases down the group | Increases down the group | Increases down the group |
| Sodium | Decreases down the group | Increases down the group | Increases down the group |
| Potassium | Decreases down the group | Increases down the group | Increases down the group |
| Rubidium | Decreases down the group | Increases down the group | Increases down the group |
Caesium is the next element in group 1, and it can be found below rubidium. You can accurately predict that it will have the lower melting point, higher density and higher reactivity than the elements above it in group 1.
Question
Can you suggest the properties of francium that come below caesium?
- lower melting point,
- lower boiling point,
- higher density,
- more reactive
Question
Radon is situated below xenon in group 0. Use the graph above to predict the likely boiling point of radon.
The actual boiling point of radon is -61.7˚C. An estimate would lie midway between -100˚C and -50˚C based on the shape of the graph.
Question
The graph shows the melting and boiling points of the first four group 7 elements. Astatine is placed below iodine in group 7. Predict the melting and boiling points of astatine and its state at room temperature.
Astatine should have a melting point of about 300┬░C and a boiling point of about 340┬░C. This means that it will be solid at room temperature.
The human body
The chemical elements of the human body, in order of % found are:
| Element | Symbol | % found in the human body | Found |
|---|---|---|---|
| Oxygen | O | 65 | All liquids, e.g., blood, tissues and bones |
| Carbon | C | 18 | Everywhere |
| Hydrogen | H | 10 | All liquids, e.g., blood, tissues and bones |
| Nitrogen | N | 10 | All liquids, e.g., blood, tissues and bones |
| Calcium | Ca | 1.5 | Bones, lungs, kidney, liver, thyroid, brain, muscles, heart |
| Phosphorus | P | 1 | Urine, bones |
| Potassium | K | 0.35 | Enzymes |
| Sulphur | S | 0.25 | Proteins |
| Sodium | Na | 0.15 | In all liquids and tissue |
| Magnesium | Mg | 0.05 | Lungs, kidney, liver, thyroid, brain, muscles, heart |
Small traces of several other elements can also be found:
Fluorine (bones, teeth), Chlorine (in body liquids), Manganese (in enzymes), Iron (in enzymes, blood)As well as minimal traces of:Aluminium, Arsenic, Bromine, Cobalt, Copper, Iodine, Lead, Lithium, Molybdenum, Selenium, Silicon , Strontium, Vanadium, Zinc
Summary
- The modern periodic table is based on Dmitri MendeleevÔÇÖs 1896 observations that chemical elements can be grouped according to common chemical properties
- the elements are arranged in order of increasing atomic number
- atomic number increases from left to right and from top to bottom
- the horizontal rows are called periods
- the vertical columns are called groups
- elements in the same group have similar chemical properties
- trends in properties of the elements in a group allow us to make predictions
- group 1 ÔÇô the alkali metals
- group 7 ÔÇô the halogens
- group 0 ÔÇô the noble gases
- the central block between groups 2 and 3 ÔÇô the transition metals
- there are 118 known elements
- the only letter not in the periodic table is the letter J
More on Chemistry
Find out more by working through a topic
- count4 of 8

- count5 of 8
- count6 of 8

- count7 of 8
