Key points
Substances can exist in three states of matter - solid, liquid and gas.
Substances can change states. To change the state of a substance energy must be transferred to, or from, the substance.
heating curveA graph showing the temperature of a substance against the amount of energy absorbed, as the substance is heated. and cooling curveA graph showing the temperature of a substance against the amount of energy transferred from it, as the substance is cooled. can be used to determine the temperature at which a state change takes place.
Video - Changes of state
Phew! On a hot day I just love an ice lolly. Ah or a liquid lolly.
I know what’s happened here! There’s been a change of state.
When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling.
These changes are reversible, too. When the gas is cooled, the vapour condenses to make it a liquid again. Cool that liquid down even more, and it will freeze to make a solid.
Liquids can freeze and melt, or evaporate and condense over and over again, without making any new compounds or changing their mass.
So, after a bit of time in the freezer, my lolly is frozen again. But have you ever wondered why this happens?
Inside a solid substance, these particles have energy in a kinetic store, making them vibrate, even though they’re held in place by strong bonds.
When you heat a substance, the energy in that kinetic store increases, making them move around faster and further until the bonds between the particles break.
The particles can now move from their fixed positions, and the substance changes state. First from solid to liquid, then if we continue heating to raise the temperature and break the remaining bonds between particles, from liquid to gas.
All substances will change state at specific temperatures, known as their melting and boiling points.
Now, I’ve been looking forward to this!
Can you answer these questions based on the video?
What are the three states of matter?
All substances will change state at specific temperatures. What are these known as?
Substances can exist as solids, liquids or gases.
All substances will change state at their melting and boiling points.
Changes of state
Many substances can exist as solids, liquids or gases, which are all different states of matterMatter (substances) can exist in three states - solid, liquid or gas.
By heating or cooling a substance, its state can be changed.
There are four main change of stateA change of a substance from one physical state (solid, liquid or gas) to another.:
melting - the process of a solid turning into a liquidOne of the three states of matter. Liquids, like water or oil, do not have a fixed shape and can flow.
freezing - the process of a liquid turning into a solidOne of the three states of matter. Solids, like ice or concrete, have a fixed shape and cannot be compressed easily.
evaporating - the process of a liquid turning into a gasOne of the three states of matter. Gases, like oxygen or helium, do not have a fixed shape and can expand to fill their container.
condensing - the process of a gas turning into a liquid

Did you know?
Liquids do not have to be heated to their boiling point to evaporate - evaporation can take place at lower temperatures too.
However, evaporation happens very rapidly once the liquid has reached its boiling point.

Energy and changes of state - melting and freezing
Heating a solid substance, like ice or copper, will initially increase its temperatureHow hot or cold something is. Temperature is most commonly measured in degrees Celsius, represented by the symbol В°C..
The energy (heat) transferred to the substance causes the particlesAll substances are made of particles. Particles could be atoms, molecules or ions.to vibrate more rapidly although the particles remain in their fixed positions, in neat, ordered rows.
If the solid is heated more, after reaching its melting pointThe highest temperature at which a substance can exist as a solid. For solid water (ice), the melting point is 0 В°C., the energy gained by the particles allows them to partly overcome the strong forces holding them in place.
The particles begin to move from their fixed positions but are still closely spaced together. The substance has melted to become a liquid.
meltingA change of state - the process of a solid changing into a liquid.is a reversible, physical change. By cooling the liquid sufficiently, it can turn back into a solid - a process known as freezingA change of state - the process of a liquid changing into a solid..
The liquid must first be cooled to its freezing point (the same temperature as its melting point).
Continuing to cool the liquid decreases the energy of the particles and they return to fixed positions - in neat, ordered rows.
Energy and changes of state - evaporating and condensing
Heating a liquid, like water or oil, also increases its temperatureHow hot or cold something is. Temperature is most commonly measured in degrees Celsius, represented by the symbol В°C. at first.
The heat transferred to the substance increases the energy of the particlesAll substances are made of particles. Particles could be atoms, molecules or ions.. This causes the particles to vibrate more rapidly and move more freely, but they remain closely spaced and randomly arranged.
If the liquid is heated further, after reaching its boiling pointThe highest temperature at which a substance can exist as a liquid. Evaporation happens rapidly when a liquid reaches boiling point., some of the particles gain enough energy to completely overcome the forces holding them together.
These particles escape from the liquid altogether and move freely, because they are no longer bound to the other particles in the liquid. These particles have evaporated.
When all the particles have escaped, the liquid has completely evaporated to become a gas.
evaporatingA change of state - the process of a liquid changing into a gas. Evaporating is also known as vaporisation. is a reversible, physical change. By cooling the gas sufficiently, it can turn back into a liquid, a process known as condensing.
The gas must first be cooled to its boiling point, after which continuing to cool the gas decreases the energy of the particles.
This causes the substance to return to the liquid state, with the particles closely spaced and in a random arrangement.

Did you know?
Solid carbon dioxide is often called dry ice. This is because heating it causes it to change directly into a gas, without becoming a liquid first – a process called sublimation.
If the gaseous carbon dioxide is cooled, it changes directly from a gas to a solid, without becoming a liquid first – a process called deposition.

Heating curves
A heating curveA graph showing the temperature of a substance against the amount of energy absorbed, as the substance is heated. can be produced by heating a substance at a constant rate and measuring its temperatureHow hot or cold something is. Temperature is most commonly measured in degrees Celsius, represented by the symbol В°C..
The diagram shows a heating curve for water.
Look through the slide show below to find out more about each stage of the heating curve.
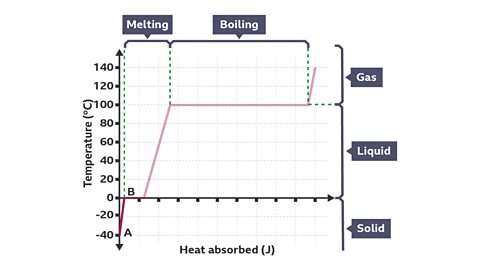
Image caption, Points A to B
The sloped section at the start of the line, between A and B, shows that the water is initially a solid (ice) at -40 В°C. As the solid ice is heated, the temperature increases until it reaches its melting point of 0 В°C.
Image caption, Points B to C
Once the ice reaches its melting point the line becomes horizontal (between B and C), showing that the temperature does not increase during melting. Instead, the energy supplied during melting enables the particles to overcome the strong forces holding them together, allowing them to move from their fixed positions.
Image caption, Points C to D
When the melting process has finished, all the ice has become liquid water. After this, the energy transferred continues to increase the temperature of the water – shown by the second sloped section of the line, between C and D.
Image caption, Points D to E
The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 В°C. Again, the line becomes horizontal (between D and E), showing that the temperature does not increase during boiling. The energy supplied during boiling enables the particles to overcome the forces holding them together, allowing them to escape completely from the liquid. When the boiling process has finished, all the water has become steam.
Image caption, Points E to F
Continuing to heat the steam will again increase the temperature – shown by the final sloped section of the line, between E and F.
1 of 5
Heating curve for sodium chloride
Not all substances melt (or freeze) at 0 В°C and boil (or condense) at 100 В°C, like water does. Different substances have different melting points and boiling points.
For example, sodium chloride (table salt) has a melting point of 801 В°C and a boiling point of 1465 В°C, as this heating curve shows:
In which section of the graph would the sodium chloride be a liquid?
The sodium chloride would be a liquid between points C and D.

Did you know?
The boiling point of water is 100 В°C at normal atmospheric pressure, at sea level. At higher altitudes, the air pressure is lower, and water boils at a lower temperature. At the top of Mount Everest, water boils at around 70 В°C.

Cooling curves
A cooling curveA graph showing the temperature of a substance against the amount of energy transferred from it, as the substance is cooled. can be used to determine the temperatureHow hot or cold something is. Temperature is most commonly measured in degrees Celsius, represented by the symbol В°C. at which changes of state occur.
A cooling curve is produced by measuring the temperature of a substance as it cools and then plotting a graph of temperature against the amount of energy transferred.
This diagram shows a cooling curve for water.

Look for the flat sections of the heating or cooling curve to find the temperatures at which the substance changes state.
Test your knowledge
Quiz
Atomic Labs game. gameAtomic Labs game
Try out practical experiments in this KS3 science game

More on Solids, liquids and gases
Find out more by working through a topic
- count3 of 4

- count4 of 4

- count1 of 4
