Pure and impure substances
Evaporation
Either evaporation or crystallisation can be used to separate a solid solute from a solution. Learn more in this KS3 Chemistry guide from Bitesize.
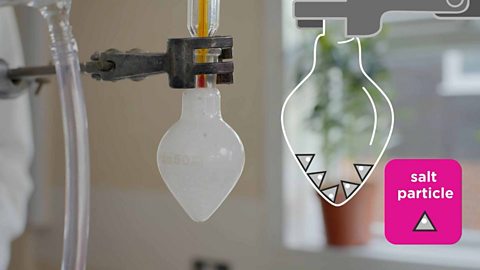
Distillation
Distillation is a separation technique is used to remove a solvent from a mixture and keep it rather than it mixing with the air and being lost.
Chromatography
Chromatography can be used to separate a mixture of soluble substances, like the pigments in ink.

Pure substances
Most materials that we use are mixtures, and just a few are pure elements or pure compounds. In chemistry, a pure substance is a single substance made of only one type of particle.

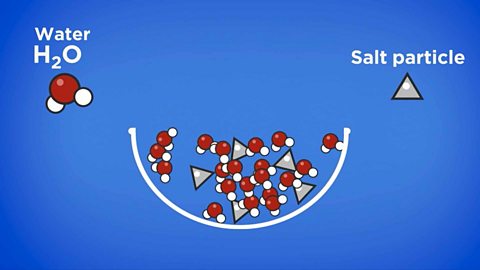
Dissolving
A solution is made when a solute dissolves into a solvent. If a substance can dissolve into a solvent, it is soluble. If it cannot dissolve, it is described as insoluble.

Diffusion
Diffusion is the movement of a substance from an area of high concentration to an area of lower concentration.

Filtration
Filtration is used to separate an insoluble solid from a pure liquid or a solution.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription