Indicators of a chemical reaction
A chemical reaction is when one or more substances change and produce one or more new chemical substances.
The substances that are involved in a chemical reaction and that are changed by it are called reactants.
The substances that are produced by a chemical reaction are called products.
Chemical reactions happen all around us (and inside us!) all the time. We might not always notice them but there are four indicators that show a chemical reaction has taken place:
- colour change
- effervescence
- precipitation
- energy (temperature) change
Colour change in chemical reactions
A colour change might take place when two substances react. It can also happen when a compound is broken down by heating it. This is called thermal decomposition.
Watch this video to see how the colour of copper carbonate changes when it is heated.
Thermal decomposition of copper carbonate
Copper carbonate is green and copper oxide is black. You can see a colour change from green to black during the reaction. The carbon dioxide produced can be detected using limewater, which turns cloudy.
Effervescence in chemical reactions
Some chemical reactions result in a product that is in a different state to the reactants.
An example of this is effervescence in which a reaction in a liquid produces bubbles of gas. Effervescence is an indicator of a chemical reaction taking place.
Watch this video to see how magnesium and dilute hydrochloric acid react to produce bubbles of hydrogen gas.
The reaction of magnesium and hydrochloric acid produces effervescence
Precipitation and chemical reactions
Some liquids react together to produce an insoluble solid. This is called a precipitation reaction and the solid formed is called a precipitate.
Precipitation reaction of lead nitrate and potassium iodide
Energy change and chemical reactions
Every chemical reaction involves a change in energy because different substances hold different amounts of energy.
During the reaction, bonds inside the substances that are reacting together must be broken and new chemical bonds must be formed in the products that are being made.
Chemical reactions can take in energy or release energy, often in the form of heat. This causes a change in temperature.
There are two different types of energy change that can take place.
Exothermic reactions
In an exothermic reaction the reactants hold more energy than the products, so exothermic reactions release energy.
This energy is most commonly a release of heat energy which would be indicated by a temperature rise.
Energy could also be released in a chemical reaction in the form of a sound or light being produced but the most exciting chemical reactions will probably have all three going on!

Exothermic reactions happen around us in everyday life. Fuels burning in combustion reactions involve energy being released. It doesnÔÇÖt matter if itÔÇÖs a small object like a match or a whole bonfire that is burning, both heat energy and light energy are given out.
Not every exothermic reaction is as exciting as a combustion reaction. When acids and alkalis react together, the energy released is not as obvious. Mixing the two solutions and stirring results in a small increase in the temperature of the reaction mixture.
Watch this video to see how an increase in temperature can be measured when magnesium reacts with hydrochloric acid.
Exothermic reaction between magnesium and hydrochloric acid
Endothermic reactions
In an endothermic reaction, the reactants have less energy than the products, so more energy is needed for the reaction to take place.
Normally, endothermic reactions take in heat. So endothermic reactions can be identified by a decrease in temperature.
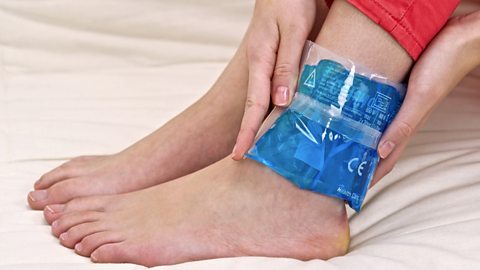
There are fewer examples of endothermic reactions in everyday life. Chemical cold packs that are used to treat bumps and sprains use an endothermic reaction to cool down. Squeezing the cold pack bursts a small inner bag allowing two chemicals to mix. The result is an endothermic reaction that cools the pack down and it can be used to stop or reduce swelling.
Watch this video to see how a decrease in temperature can be measured when barium hydroxide and ammonium chloride react.
Endothermic reaction between barium hydroxide and ammonium chloride