Key points
- Everything in the known universe is made up of the elements found on the periodic table. There are over 100 different elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table., which are made up of atoms.
- Particle diagrams are used to help explain elements, compoundA pure substance made from two or more elements which are chemically bonded in a fixed ratio. and mixtureWhen two or more compounds or elements are present without being chemically bonded together..
- Some elements exist as individual atoms, but some bond together to form moleculeTwo or more atoms which are strongly bonded together. The smallest particle of a substance that has all of the physical and chemical properties of that substance. of atoms of the same element.
Video
Watch this video to find out all about elements, compounds and mixtures.
Everything we can see and touch, and quite a lot that we canвҖҷt as well, is made of tiny particles called atoms.
Some substances, like particles of this iron, contain only one kind of atom.
Iron is an element. It is made of only one type of atom: iron atoms.
Sulfur is another element. It contains only sulfur atoms, and nothing else, so it is pure.
When we mix two different pure substances together, like this, itвҖҷs a mixture.
This is now a mixture of the elements iron and sulfur.
The different elements are not joined together.
We can still separate the elements in the mixture. The iron can be removed using a magnet.
When the iron and sulfur are heated togetherвҖҰвҖҰatoms of the two elements are now joined together by chemical bonds.
The iron can no longer be removed by a magnet because itвҖҷs bonded to the sulfur.
Iron sulfide, a new substance, has been formed because the iron and sulfur atoms are now chemically bonded together.
The new substance is called a compound.
Other compounds are made from different combinations of atoms, like waterвҖҰ carbon dioxideвҖҰ and table salt.
And thatвҖҷs it! Nearly everything in this room, in your room, and in the entire universe, is made of: elements with one kind of atom, compounds containing different types of atoms chemically bonded together and mixtures of different elements and compounds together.
Why could the iron be separated easily from the sulfur before they were heated together, but not afterwards?
Iron is a magnetic element, so it can be separated from a mixture using a magnet. But after the iron and sulfur have been heated, they react to make the compound iron sulfide. You cannot easily separate an element from a compound.
Elements
An element is a pure substance that cannot be broken down into any other substances. An element is made from just one type of atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons., and examples include oxygen, hydrogen and iron. Some elements exist as individual atoms, but some exist as molecules.

Did you know?
Most of the elements are metals. Aluminium (Al) is used to make bike frames because it is light. It is also used to make kitchen foil. Copper (Cu) is used to make electrical wires because it is a very good conductor of electricity.

How many chemical elements do you think there are?
The ancient Greeks thought that there were just four elements - earth, air, fire and water. However, we now know that there are actually 118 chemical elements. Everything on Earth and in the solar system is made from these elements.
A few of these elements can be found pure in a natural state. For example, graphite and diamond can be found in rocks in the EarthвҖҷs crust, and they are forms of the element carbon. Gold is an example of a metal element that can also be found in rocks.
The air we breathe is mostly made from nitrogen and oxygen, both of which are elements.

All the atoms in a copper wire are the same. These are different to the atoms in aluminium foil which are all the same as each other.
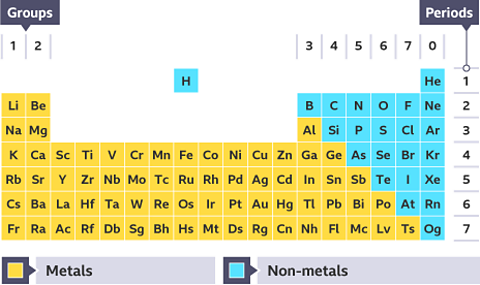
The periodic table
There are 118 chemical elements. They are listed on the periodic tableA table which lists all of the chemical elements and arranges them in a way that is useful. It allows us to spot patterns and make predictions about other elements. in a specific order.
The periodic table can be divided into metals and non-metals. Metals are found on the left and in the middle, whereas non-metals are on the right. There is a zig-zag diagonal line dividing metals and non-metals in the periodic table. Hydrogen doesnвҖҷt fit into this grouping and is placed over the table, this is because of Hydrogen's atomic structure.
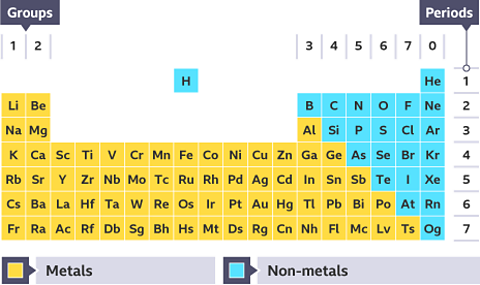
- Hydrogen is the first element listed in the periodic table. We use the symbol H for hydrogen. It is an explosive gas which burns with a very clear вҖҳsqueaky popвҖҷ when a burning splint is inserted into a test tube containing it.
- Helium is the second element in the periodic table, and has the symbol He. Helium is also a gas, but is very different from hydrogen because it doesnвҖҷt take part in any chemical reactions. Helium is used for party balloons because it is lighter than air.
The first 94 chemical elements, up to plutonium, occur naturally on Earth and elsewhere in the universe. The heaviest elements are made by humans using nuclear reactions, but these elements cannot be seen in this version of the periodic table.

Did you know?
Scientists Marie and Pierre Curie discovered two elements: radium and polonium. For these discoveries, Marie Curie received a Nobel prize.

Where are the metal elements found on the periodic table?
To the left and in the middle.
Particle diagrams - elements
Particle diagrams can be used to show the atoms of elements. All of the atoms in a particle diagram must be the same colour and size.
A particle diagram can represent a solid element, a liquid element or a gaseous element.
1. A solid element
The particles are close together and arranged in a regular way.
2. A liquid element
The particles are close together and arranged in a random way.
3. A gaseous element
The particles are far apart and arranged in a random way.
Molecules
Some non-metal elements are made from atoms which are bonded into clusters called molecules.
For example, oxygen is a gaseous element made from molecules.

A molecule is made up of 2 or more atoms. These can be the same type or different.

This diagram shows helium. Is helium a solid, liquid or a gas? Is it made up of atoms or molecules?
Helium is a gas made up of atoms. Each particle is on its own and they are far apart, arranged in a random wayвҖҰ If it was made of molecules, they would be in clusters of two or more.
Compounds
A compound is a pure substance that is made from more than one element. In a compound, elements are chemically bonded together, which makes it very difficult to separate them.
When a compound is made, the atoms of the elements bond together in a fixed ratioAвҖҜratioвҖҜshows how much of one thing there is compared to another.. This means that each compound can be represented by a chemical formula.
For example, the formulaA formula is made up of the symbols for one or more element and a subscripted number to show how many atoms of each element are bonded together. of water is ұбвӮӮO and the formula of carbon dioxide is °д°ҝвӮӮ.

To break apart the elements in a compound, a chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. must take place, for example, electrolysisSplitting up a liquid or dissolved compound into its elements using electricity. or thermal decompositionBreaking down a compound into its elements, or simpler compounds using a high temperature. . However, when elements make a mixture, then through a physical process such as filtrationA technique used to separate an insoluble solid from a pure liquid or a solution. or crystallisation, it is possible to separate them.
Compounds are not found on the periodic table. For example, water isnвҖҷt on the periodic table because it is a compound, not an element. Water is made from the element hydrogen bonded to the element oxygen.
Particle diagrams - compounds
A particle diagram for a compound will show more than one type of atom.
For example:
ұбвӮӮO
Water molecules are made up of two elements - hydrogen (white atoms) and oxygen (red atoms).
Water has a specific ratio of two hydrogen atoms to one oxygen atom.
°д°ҝвӮӮ
Carbon dioxide molecules are made up of two elements - carbon (black atoms) and oxygen (red atoms).
Carbon dioxide has a specific ratio is one carbon atom to two oxygen atoms.
Non-metals
Compounds made from non-metal elements that are bonded together usually form molecules.
Compounds including both a metal element and a non-metal element bonded together do not form molecules.
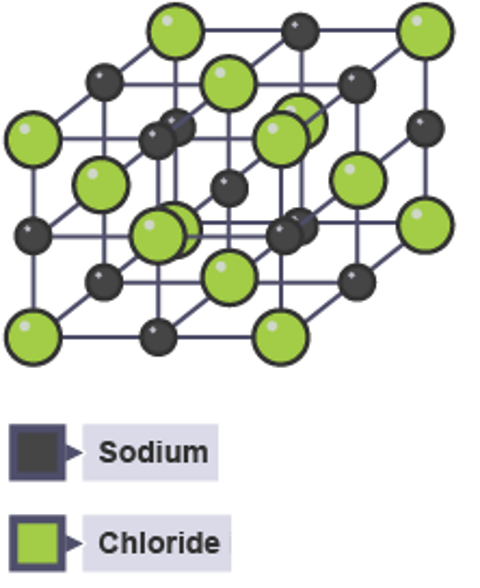
Their diagrams look quite different, this is called a lattice.
If this 3D particle diagram for sodium chloride was shown on a large scale, it would show the same number of metal sodium atoms (grey) as chlorine atoms (green), because the formula is NaCl.

Mixtures
A mixture is formed when two or more elements or compounds are present without being chemically bonded together.
The substances which have been mixed are not present in specific amounts or ratios like they are in a compound, e.g. two hydrogen atoms for each oxygen atom in water. They can be in any combination, e.g. for a mixture of sand and water you could have any amount of sand with any amount of water.

In a mixture, the two ingredients can be separated using physical processes, without chemical reactions. This is because they are not chemically bonded together.
Here are some examples:
- A mixture of sand and water can be separated using filtrationA technique used to separate an insoluble solid from a pure liquid or a solution..
- A solution of salt and water can be separated using crystallisationA separation technique used to obtain large crystals of a solute from a solution. or distillationA separation technique which separates the solvent from a mixture, (usually a solution or two liquids with different boiling points). Distillation involves heating the mixture to boil off one of the parts and then condensing the vapour..
- A mixture of iron filings and sulfur powder can be separated using a magnet.

Particle diagrams - mixtures
A particle diagram of a mixture can include atoms and molecules, but they are not bonded together.
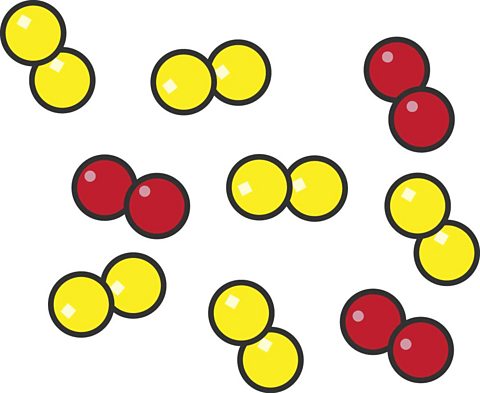
This particle diagram shows air.
- Air is a mixture which is made mainly of nitrogen molecules (yellow) and oxygen molecules (red).
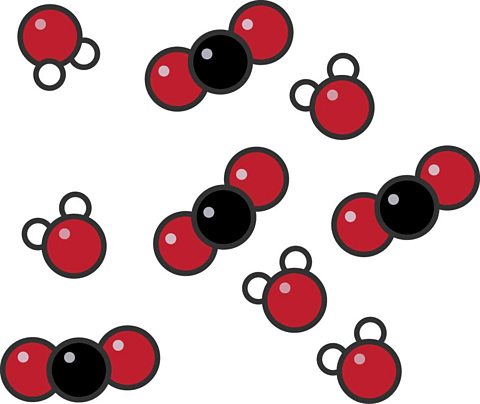
This particle diagram shows a mixture made up of water and carbon dioxide.
- The water molecules each have two white hydrogen atoms and one red oxygen atom (ұбвӮӮO).
- The carbon dioxide molecules each have one black carbon atom and two red oxygen atoms (°д°ҝвӮӮ).
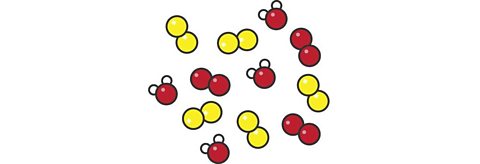
Describe what this particle diagram represents. Is it an element, compound or mixture?
ItвҖҷs a mixture. There are three substances in the mixture. The red and yellow molecules represent two elements and there is also a compound that is made from one red atom and two white atoms.
Test your knowledge
Quiz
Test questions
Whereabouts on the periodic table are the non-metals found?
On the right.
The non-metal elements are found on the right-hand side of the periodic table, and metals on the left and in the middle.
Which technique is most likely to be used to separate the elements which are bonded together in a compound?
Thermal decomposition.
Thermal decomposition can be used to break down some compounds into elements. Simple techniques like filtration and evaporation will not work, but they can be used for separating mixtures.
Teaching resources
Need extra resources for your physics and chemistry lessons? In this seven-part series, materials scientist Mark Miodownik takes viewers on a journey into the inner world of metals, ceramics and plastics.
ҙуПуҙ«ГҪ Teach has thousands of free, curriculum-linked resources to help deliver lessons - all arranged by subject and age group.
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Atoms, elements and compounds
Find out more by working through a topic
- count2 of 8

- count3 of 8
- count4 of 8

- count5 of 8
