Key points
- A solution is made when a soluteThe solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. For example, the main solute in sea water is sodium chloride. dissolves into a solventThe liquid in a solution which dissolves the solute. For example, the solvent in sea water is water..
- If a substance can dissolveThe process when a solute is mixed with a solvent and the solute breaks into much smaller particles and spreads out. into a solvent, it is solubleA solid is soluble if it can dissolve into a specific solvent. For example, salt and sugar are both soluble in water. . If it cannot dissolve, it is described as insoluble.
- Heating, stirring and using fine powders are all ways to speed up dissolving.
When sugar is added to hot water and stirred, it seems to disappear. What has really happened to it?
It has dissolved. The sugar crystals break into tiny particles which are too small to see and spread out through the hot water.
Video
Watch this video to find out about how solutionA mixture made when a solute (usually a solid) dissolves into a solvent (a liquid). Sea water is a solution of salt dissolved into water. are made from a solute and a solvent.
Solubility with Jon Chase
Jon: I've got a question for you guys. Do you think I can fit this length of polystyrene into this jar?
Crowd: Yes… No.
Jon: No? Yeah? I like that – you lot have got some faith in me! Excellent.
Right, this is what we're going to do…
We're going to take this length of polystyrene and stick it into this Pyrex jug. But we're going to use something to help us do it. It's this stuff. It's called propanone, or acetone. We're going to dissolve this polystyrene into this. And when we dissolve it in, this will be called the solute, and this stuff, that does the dissolving, will be called the solvent. And when they're mixed together, they will be called a solution.
You add a bit of the solvent…
Something's happening.
GIGGLING
Slowly but surely, it's going in. So remember, acetone's the stuff that you've actually got in nail varnish remover.
Aw, yeah!
Swill that around.
Crowd: Hurray! One, two, three… Magic!
Jon: Oh, yes. So there we have it, a whole polystyrene rod fitted into a little Pyrex jar.
Hi. Can I have two cups of tea, please?
Barista: Sure.
Jon: Solubility is a measure of how much solute can dissolve in a solvent.
The solubility of a solute in a solvent changes with temperature. And importantly, it depends on whether the solute is a gas or a solid. So, let's look at solids first.
Here we have two identical hot cups of tea. And we want to see how much sugar can be held in solution in these hot cups of tea. When no more sugar can dissolve, the solution is said to be saturated.
I think that's getting just about saturated now. But the situation changes when the liquid is cooled down. Luckily, I've got a bit of dry ice here, and that should do the job perfectly. Dry ice is at a temperature of minus 78 degrees centigrade, so it's going to cool the water down.
In this one you can see a tiny bit of sugar has crystallised at the bottom because it's a bit cooler. But this one, look how much sugar is actually in the bottom. The sugar behaves like most solids - the solubility increases as the temperature of the solvent does.
What's interesting about gases is that they behave in the opposite way to solids. The solubility of gases decreases as the temperature increases.
I'm going to show you a neat trick. Check out these ice cubes. One sets lovely and clear, but the other one's pretty cloudy. It's all down to the solubility of gases in a liquid. So to make cloudy ice cubes, all we need to do is, take tap water and put it straight in. But if we want our ice cubes to be clear, you have to boil the water first. And here's why boiling the water makes a difference.
At room temperature, the water contains a certain amount of dissolved gases from the air. The water straight from the tap creates cloudy ice cubes because these gases that were dissolved in the water form tiny bubbles in the ice.
By heating the water to boiling point, we have decreased the solubility of the dissolved gases. They come out of the solution as bubbles and the remaining water has less gases dissolved, so is less cloudy.
For a solution, the solubility of gases decreases as we increase the temperature.
What happened to the solubility of sugar when the temperature of the tea was hottest?
The solubility increased with temperature. The hotter the water, the more sugar can dissolve.
What is a solution?
A solution is made when a substance dissolveThe process when a solute is mixed with a solvent and the solute breaks into much smaller particles and spreads out. into a liquid. The liquid is called the solvent. The substance that has been dissolved is called the solute.
A solution can also be called a mixture.
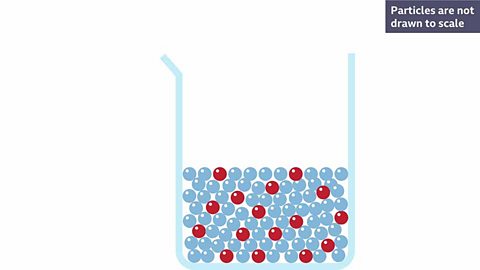
When a solid dissolves, it breaks down into smaller particles that spread out through the solvent.
Example

Salt dissolves when it is stirred into water. In sea water, the water is the solvent and the salt is the solute.

Coloured and colourless solutions
When a white solid dissolves, it makes a colourless solutionA mixture made when a solute (usually a solid) dissolves into a solvent (a liquid). Sea water is a solution of salt dissolved into water., for example, salt or sugar in water.
When a coloured solid dissolves, it makes a coloured solution, for example, copper sulphate dissolves to makes a blue solution.
Saturation
There is a limit to the mass of soluteThe solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. For example, the main solute in sea water is sodium chloride. that will dissolve in a particular volume of the solventThe liquid in a solution which dissolves the solute. For example, the solvent in sea water is water.. When no more solute dissolves, the solution is saturated.
When copper sulfate is added to water to make copper sulfate, which substance is the solvent, and which is the solute?
Copper sulfate is the solute. Water is the solvent.
How do particles behave in a solution?
What happens to particles of a solid solute when it dissolves?
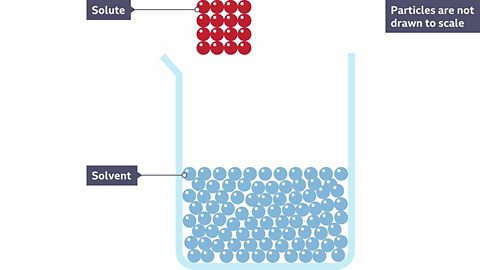
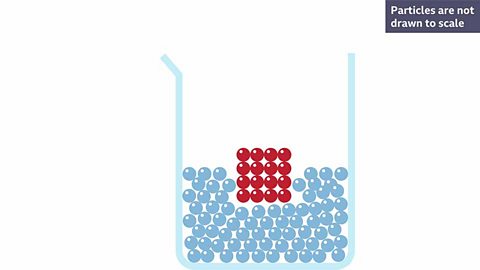
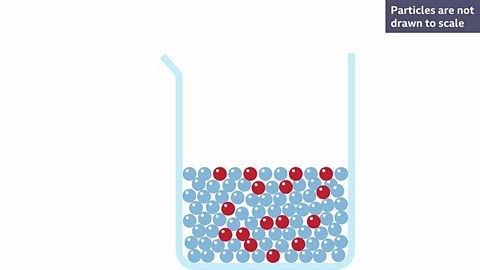
A red solute is dissolved into a colourless solvent making a pink solution. Why do you think it is pink?
Because the red solute has broken apart and the particles are now spread throughout the solvent.
Insoluble or soluble?
If a solid does not dissolve in a specific solvent, it is insolubleA solid is insoluble if it does not dissolve in a specific solvent. For example, wax is insoluble in water. in that solvent. Some examples include:
- Wax is insoluble in water.
- Chalk is insoluble in water.
- Salt is insoluble in propanone.
- Polystyrene is insoluble in water, but soluble in propanone.
When an insoluble white powder is stirred into a colourless liquid it does not dissolve. At first the liquid will appear to turn white, but after a few minutes the liquid will return to colourless as the powder settles on the bottom of the container.

Did you know?
Chalk is insoluble in water. If chalk is added to water and stirred, the chalk will eventually settle at the bottom of the glass.

The solubility of gas
solubilityA measurement of how soluble a solute is in a specific solvent. If the solubility is high, it means that the solute is very soluble in the solvent. measures how much solute can dissolve in a volume of solvent at a specific temperature. For example, sugar has a higher solubility in water than salt. Therefore, more sugar than salt can dissolve in a volume of water at a specific temperature.
You can speed up dissolveThe process when a solute is mixed with a solvent and the solute breaks into much smaller particles and spreads out. a solid by:
- Heating up the solvent. The solubility of a solid solute usually increases when the temperature increases.
- Stirring.
- Using fine powder rather than large pieces of solute.
The solubility of a gas usually decreases when the temperature increases.
For example, fizzy drinks have lots of carbon dioxide dissolved in them when they are cold. Fizzy drinks warm up quickly in your mouth and release bubbles of carbon dioxide gas.
Working scientifically
Planning an experiment
Often experiments always involve things that can change, known as variables. Variables need to be identified, so they can then either be changed or controlled.
Plan an experiment to investigate one of the factors that affects solubility.
Make sure that you only change one variable, independent variableThe variable that is altered during a scientific experiment. and keep the others constant for a valid investigation.
What will you measure, known as the dependent variableThe variable being tested or measured during a scientific experiment.?
Write your method as a numbered list of brief instructions.
When investigating how temperature affects the solubility of salt in 100 cmв‚ѓ of water, what would be the independent variable, the dependent variable and the control variables?
- The independent variable would be the temperature of the water.
- The dependent variable would be the amount of salt you can dissolve in 100 cmВі of water - ideally measured in grams.
- The control variables that would need to be kept the same are the size of the salt crystals and the speed of stirring.
Find out more about planning an experiment.
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Pure and impure substances
Find out more by working through a topic
- count6 of 7

- count7 of 7

- count1 of 7
- count2 of 7